Lecture 12: Making the Sun
Shine
Readings: Sections 18-1, 18-4 and Box 18-1
Key Ideas
Stars
shine because they are hot—need an internal energy source to stay hot
Kelvin-Helmholtz
Mechanism
Energy
from Gravitational Contraction
DoesnÕt
work in the Sun now, but is important during the early stages of a starÕs life
Nuclear Fusion
Energy
Energy
from fusion of 4 hydrogen atoms into 1 helium atom
Proton-proton
nuclear reaction chain
CNO
nuclear reaction chain
Why do stars shine?
Stars shine because they are
hot
Emit light with a
roughly thermal (blackbody) spectrum
Internal heat
ŌleaksĶ out of their surfaces
Luminosity=rate of energy
loss
To
stay hot, stars must make up for the lost energy, otherwise they would cool and
eventually fade out.
Case
Study: The Sun
Question
How
long can the Sun shine?
Need
two numbers
How
much internal heat is there in the Sun?
How
fast this heat is lost (Luminosity)
![]()
Sources
of Energy
In
the 19th century, two energy sources were known:
Chemical
Energy
Burning
of oil or wood by oxidation
Chemical
explosives
Gravitational
Energy
Water
running downhill to power a mill
Heat
from meteorite impacts
The
Age Crisis: Part 1
The
most powerful chemical reactions could work for only a few thousand years
Meteorites
could work for about 1 million years
But:
Geologists estimated that the Earth was at least a few 10s to a 100s of million
years old
Logical
Inconsistency:
How
can the Earth be older than the Sun?
Kelvin-Helmholtz
Mechanism
Energy from Gravitational Contraction of the Sun
As gas contracts, its temperature rises as energy
stored as gravitational potential energy is released
This energy is radiated into space, cooling the star.
Gravity keeps contracting the star, more and more energy is radiated away. When
the star has completely collapsed, youÕve gotten all the energy you can from
the Kelvin-Helmholtz mechanism.
Predicts that the SunÕs size will change with time
(though really slowly).
The
Age Crisis: Part II
Late
1800s:
Kelvin
estimated the Sun could shine for about 30 million years
Geologists estimated that the Earth is at least 2
billion years old, based on measuring the amount of uranium that had decayed to
lead in rocks.
In
an interesting twist, the phenomenon of radioactivity, which sealed the
rejection of the Kelvin-Helmholtz mechanism, also started providing the clues
that pointed out the real answer.
Nuclear
Energy
1896
Rntgen & Becquerel discover radioactivity
1905
Einstein demonstrates equivalence of Mass & Energy. Conservation says that
the sum
of matter and energy does not change.
1920s
Eddington noted that 4 protons have 0.7% more mass than 1 Helium nucleus
(2p+2n)
If 4 protons fuse into 1 Helium nucleus, the remaining
0.7% of mass is
converted to energy.
Payne-Gaposhkin showed that the SunÕs atmosphere was
mostly H.
Fusion Energy
Fuse 1 gram of hydrogen into
0.993 grams of helium
Leftover 0.007 grams is
converted into energy
![]()
Enough energy to lift 64,000
Tons of rock to height of 1 km.
The Age Crisis: Averted
Luminosity of the Sun is
about 4x1033 erg/sec (4x1026watts)
Must fuse about 600
million tons of H into He each second
Converts about 4
million tons of matter into energy each second
Sun contains about 1021
million tons of H, but only 10% is hot enough for fusion
Fusion Lifetime is about 10
billion years. The Earth is 4.5 billion years old, so the Sun has enough energy
to keep going for about 5 billion years. Phew!
Nuclear Fusion 101
Gravity unimportant, the
forces that are important are the strong nuclear, the electromagnetic, and the
weak nuclear force. Weak force at work when neutrons change into protons and
vice versa.
Nuclear particles
Proton (positively charged)
Neutron (neutrally charged)
4He and bigger
particles
Not all possible combinations
of neutrons and protons exist
5He
doesnÕt
2He
doesnÕt
and many others
Fusion==combining positively
charged nuclei together
Electromagnetic Force
Like charges repel
Opposite charges
attract
So trying to put protons
together is difficult. Proton with 4He is even tougher.
BUT strong force, which binds
protons and neutrons together, is stronger than electromagnetic repulsion.
BUT strong force is short
range (<10-15 meters), so the protons need to get really close
together before they will stick
BUT protons donÕt like to get
close together. They need to be traveling fast enough so the repulsive force
canÕt stop them. Remember that temperature measures the speed of particles in a
gas. Therefore what we need is a high temperature.
Rate of nuclear fusion
depends on temperature
Proton-Proton
Chain
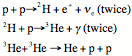
note
that the atoms are all completely ionized. IÕve dropped the + for
convenience.
The
Bottom Line
Fuse
4 protons (1H) into one 4He nucleus plus the following reaction
by-products
2
photons = Energy
2
positrons (positive electrons, will annihilate and produce more energy)
2
neutrinos (ne) that leave the Sun carrying energy
Additional
Details:
Protons
do not just collide with protons in the center of the Sun. There are other
nuclei floating about and protons can collide with these. These lead to the
pp-II and pp-III chains. These reactions also release neutrinos, neutrinos that
have higher energy than neutrinos from the pp-I chain.
Test:
Solar Neutrinos
Question:
How do we know that fusion is occurring in the core of the Sun?
Answer:
Look for the neutrinos created by the p-p chain
What
are Neutrinos?
Weakly
interacting neutral subatomic particles
Very
nearly massless
Travel
very near the speed of light
Interact
with matter via the weak nuclear force
Can
pass through lead 1 parsec thick!
Neutrinos
created by nuclear fusion in the SunÕs core would stream out of the Sun, not
interacting with the protons, electrons, helium atoms, etc. in the Sun. This is
good! They also only rarely interact with detectors on Earth. This is bad!
The
Sun emits about 1033 neutrinos/sec
(FYI, humans emit about 3x108 neutrinos/day
from the decay of radioactive potassium)
Every
second we have many neutrinos pass through our bodies: 400,000 billion from the
Sun, 50 billion from the Earth and 100 billion from nuclear power plants. This
is not dangerous, because the neutrinos do not interact.
Detection
of neutrinos is hard
Need
massive detectors
Work
deep underground to shield out other radiation
Homestake
Mine experiment
100,000
gallons of tetrachloroethylene, a common cleaning fluid.
Very
rarely, a solar neutrino will change a 37Cl into a 37Ar
atom.
Set up your tank, wait a few months, then find the few
dozen 37Ar atoms in your tank that has 1030 atoms in it.
Calculate the solar neutrino flux!
Results:
We
detect neutrinos from fusion in the Sun, with the expected energies, in all of
the experiments to date.
The
CNO cycle
In
stars with central temperatures hotter than the SunÕs, H is fused into He using
carbon, nitrogen and oxygen nuclei as catalysts. The hotter temperatures mean
that the large repulsion between the 6-8 protons and the proton can be
overcome. And there is no more annoying step where you need to proton to change
into a neutron as the moment the two protons are interacting.
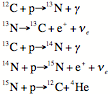
Note
that you start with 12C, add 4 protons, and end up with a 12C
and a 4He.
These
reactions happen at higher temperatures because the repulsive force increases
as the number of protons in the nuclei increases, and the nuclei have to be
moving faster to overcome the repulsion.
We
will discuss other stages of nuclear fusion (such as helium fusing into carbon)
later in the course.