|
Astronomy 141
Life in the Universe
Prof. Scott Gaudi
|
Lectures 3: & 4 Basic Physical Concepts
Key Ideas
-
-
- Scientific Notation, Units and prefixes
- The Universe is governed by four forces
- Matter is made up of atoms and electrons
- Light is electromagnetic radiation.
- Luminosity is intrinsic, Brightness is apparent
- Interaction of light and matter
- Temperature is a measure of internal energy
- Spectra are diagnostic indicators of composition
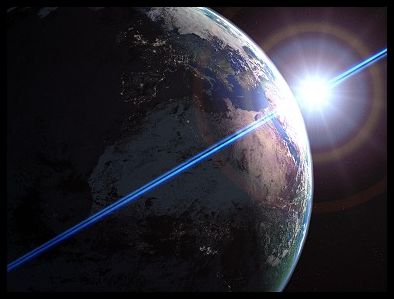
Scientific Notation
- Very large and very small numbers are unwieldy.
- We use scientific notation as a compact way of expressing large and small numbers.
- Example:
Scientists Use the Metric System (and so will we!)
- Length: meters
- Mass: kilograms
- Time: seconds
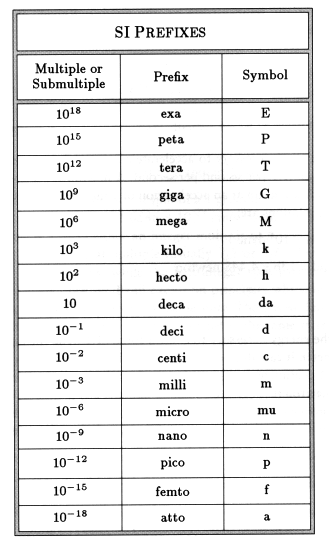
Standard Prefixes
Astronomical Units of Length
- Astronomical Unit (AU): Mean distance from the Earth to the Sun
- Light Year (ly): Distance light travels in one year.
- Parsec (pc): Distance at which a star's parallax would be one arcsecond
The Four Forces
- Strong Force: binds nuclei
- Weak Force: radioactive decay
- Electromagnetic: binds atoms, light, magnetism
- Gravity: binds the solar system, galaxies, universe
Atomic Structure
- Nucleus: Protons(+) and Neutrons
- Electrons(-): Orbit the nucleus
Elements
Each element has a different number of protons in the nucleus.
- One proton: Hydrogen
- Two protons: Helium
- Three protons: Lithium
- .
- .
- .
Ten Most Abundant Elements
1. Hydrogen
2. Helium
--------------
3. Oxygen
4. Carbon
5. Neon
6. Nitrogen
7. Silicon
8. Iron
9. Magnesium
10. Sulfur
Only Hydrogen and Helium were produced in appreciable amounts in the big bang.
The rest of the elements were produced by stars.
Carbon, Hydrogen, Oxygen, and Nitrogen play important roles in the chemistry of life.
Isotopes
- Same number of protons, but different number of neutrons.
- Chemistry is the same, but radioactivity different (governed by weak force).
- Important for age dating.
Radioactivity
- Longevity of a species is quantified by its Half-Life
- The amount of time when half of the atoms decay.
Electromagnetic radiation
Light is electromagnetic radiation
- Waves or particles.
Speed of light is constant
- speed = frequency times wavelength
- Low energy = low frequency = long wavelength
- High energy = high frequency = short wavelength
Luminosity versus Brightness
- Luminosity: total energy (photon) output of a source.
- Brightness: apparent brightness from a distance.
- Luminosity is independent of distance.
- Brightness follows an inverse square law:
- A light source that is 10 times further away will be 100 times fainter
- A light source that is 2 times closer will be 4 times brighter.
Interaction of Light and Matter
- Matter can transmit light.
- Matter can reflect light.
- Matter can absorb light.
- Matter can emit light.
Temperature
- Temperature is a measurement of the internal energy content of an object.
- Gases - higher temperature means average speed per atom or molecule.
Spectrum
- A spectrum is the distribution of photon energies emitted by a light source:
- Number of photons of each energy that are emitted.
- Spectra are observed by passing light through a spectrograph:
- Breaks light into its component colors.
- Uses either prisms or diffraction gratings.
Continuous Spectra
- Most objects can be described as Blackbodies.
- Blackbodies are characterized by their Temperature.
- Blackbodies emit at all wavelengths (continuous spectrum)
- Energy emitted depends on Temperature.
- Peak wavelength depends on Temperature.
- Stefan-Boltzmann Law
- : Energy emitted per second per area by a blackbody with Temperature (T):
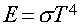
- sigma is Boltzmann's constant (a number).
- In Words:
- "Hotter objects are Brighter at All Wavelengths"
- Note the relative brightnesses at each wavelength for the three
different-temperature blackbodies above.
- Wien's Law: Relates peak wavelength and Temperature:
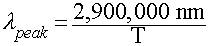
- In Words:
- "Hotter objects are BLUER"
- "Cooler objects are REDDER"
- Note the wavelength of peak brightness for the three different-temperature blackbodies above.
Absorption-Line Spectrum
- Light from a continuous spectrum through a vessel containing a cooler gas shows:
- A continuous spectrum from the lamp crossed by of dark "absorption lines" at particular wavelengths.
- The wavelengths of the absorption lines exactly correspond to the wavelengths of emission lines seen when the gas is hot!
- Light is being absorbed by atoms in the gas.
- Each element and molecule has a characteristic line spectrum:
- The particular wavelengths of the absorption or emission lines of each element and molecule are unique
- Essentially a fingerprint of the element or molecule
- Reflects the detailed structure of the atom.
- Depends on the number and arrangement of electrons in orbit around the nucleus.
- From the an object's spectrum, we can learn which atoms and molecules are present, and in what proportions.
See A Note about Graphics to learn
why some of the graphics shown in the lectures are not reproduced with
these notes.
[
Return to the Astronomy 141 Main Page
|
Unit 1 Page
]
Copyright © Scott Gaudi All Rights
Reserved.