

|
Astronomy 161:
An Introduction to Solar System Astronomy
Prof. Richard Pogge, MTWThF 9:30
|
Lecture 23: Worlds Within: Atoms
Key Ideas:
Atoms are composed of
- a nucleus of protons & neutrons
- orbiting electrons
Chemical Elements and Isotopes
Radioactivity
Four Fundamental Forces:
- Gravitational & Electromagnetic
- Strong &Weak Nuclear Forces
Atoms
Ordinary matter is found primarily in the form of atoms.
Range of ordinary matter:
- Fundamental Particles (quarks & leptons)
- Subatomic Particles (protons, neutrons, & electrons)
- Single Atoms (hydrogen, helium, gold, etc.)
- Simple Molecules (O2, H2O)
- Macromolecules (DNA, complex polymers)
- Macroscopic Objects (rocks, people, planets, etc.)
On the Earth, matter is rarely in the form of fundamental or subatomic
particles, but most often in the form of simple atoms and larger.
Atomic Structure
Nucleus of heavy subatomic particles:
- proton: positively charged
- neutron: uncharged (neutral)
Cloud of Electrons orbiting the Nucleus:
- negatively charged particles
- masses are 1/1836th the mass of a proton
Atoms are mostly empty space:
- Only 1 part in 1015 of the volume is occupied.
- Volume is threaded by electromagnetic fields.
A simple, yet familiar cartoon:
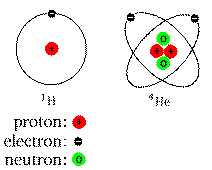
Real atoms don't look like this, but it can help you visualize the
locations and numbers of the different components (nucleus and
electrons).
Chemical Elements
Distinguish atoms into Elements by the total
number of protons in the nucleus.
Atomic Number:
- 1 proton = Hydrogen
- 2 protons = Helium
- 3 protons = Lithium ... and so on
Number of electrons = Number of protons
All elements are Chemically Distinct
Top Ten Most Abundant Elements
- 10) Sulfur
- 9) Magnesium
- 8) Iron
- 7) Silicon
- 6) Nitrogen
- 5) Neon
- 4) Carbon
- 3) Oxygen
- 2) Helium
- 1) Hydrogen
Known Elements
118 elements are currently known:
- 87 are metals
- 11 are gasses
- 2 occur as liquids (Bromine & Mercury)
- 26 are radioactive
- 25 are made only in particle accelerators
In addition, each element can have a number of isotopes.
The newest possible member of the table of the elements, with atomic
number 118, was jointly announced by Russian and American scientists who
briefly made this unstable atom by colliding atoms of cesium (20
protons) and Californium (98 protons) in a particle accelerator.
Isotopes
A given elements can have many Isotopes
- Same number of protons.
- Different number of neutrons.
Again, a simple cartoon:
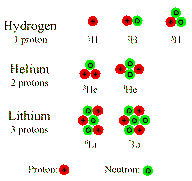 [Click on image for full-size version (17Kb GIF)]
[Click on image for full-size version (17Kb GIF)]
Example:
- 12C has 6 protons and 6 neutrons
- 13C has 6 protons and 7 neutrons
- 14C has 6 protons and 8 neutrons
All isotopes of a given element are chemically identical, but they have
different masses.
Radioactivity
If a nucleus has too many or too few neutrons, it becomes
unstable to radioactive decay
Examples:
3H (1p+2n) -> 3He (2p+1n) + e- + neutrino
14C (6p+8n) -> 14N (7p+7n) + e- + neutrino
(basis of radioactive carbon dating)
Free neutrons are also unstable:
n -> p + e- + neutrino
All of the above are examples of "beta decay", in which one
of the neutrons turns into a proton, shedding its excess mass by
spitting out an electron and a neutrino.
Radioactive Half-Life
Radioactive decay is a random process.
The activity is measured in terms of the Half-Life of the
isotope:
- Time for half of the radioactive isotopes in a sample to decay.
- The more "radioactive" the isotope, the
shorter the half-life.
Examples:
3H -> 3He + e- + neutrino : half-life = 12.26 years
14C -> 14N + e- + neutrino : half-life = 5730 years
n -> p + e- + neutino : half-life = 12 minutes
Note:
Radioactivity is an example of a random process, and the
half-life of an element is a strictly statistical measurement
of its radioactivity. This makes it somewhat unfamiliar to most people
at first encounter.
An every-day analogy that will help you to better understand both random
processes and half-lives is to consider popping popcorn. You never pop
popcorn one kernel at a time, you always make a batch (i.e., you work
with a "sample" made up of many kernels). Similarly, popcorn
popping is a random process like radioactive decay: you don't know when
any particular kernel will pop. Some will pop right away, and some never
seem to pop.
A way to measure the "activity" of the popcorn is to measure
the time it takes for half of the kernels to pop on average. We
would call this time the "half-life" of the popcorn. Very
poppy popcorn that pops up quickly has a short half-life, while popcorn
that takes a long time to pop has a long half-life. Regular sweet corn
is "inactive", in the sense that no matter how long you wait,
it never pops.
Fundamental Forces of Nature
All interactions in nature are governed by four "fundamental"
forces:
- Gravitational Force
- Electromagnetic Force
- Strong Nuclear Force
- Weak Nuclear Force
Gravitational Force
Gravitation binds masses over long distances.
- Long-range attractive force
- Weakest force of nature.
- Obeys the Inverse Square Law of distance:
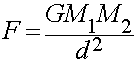
Electromagnetic Force
Electromagnetic force acts between charged particles
- Like charges repel each other
- Opposite charges attract each other
Long-range, inverse-square law force:
- Binds electrons and protons into atoms
- Binds atoms together into molecules
The Electromagnetic force is very strong: approximately 1039
times stronger than Gravity.
Strong & Weak Nuclear Forces
Short-range forces (<10-15m) in atomic nuclei
Strong Force:
- Binds quarks insde protons & neutrons.
- Binds protons & neutrons into nuclei.
- Strongest force of nature.
Weak Force:
- Responsible for radioactivity (turns neutron into a
proton, electron, and neutrino)
- Second weakest force.
The Interplay of Forces
Gravity rules on the largest scales:
- Binds massive objects together
- Mediates orbital motions
Electromagnetism rules on atomic scales:
- Binds electrons to protons, atoms to atoms
- Mediates chemical reactions
Strong & Weak Forces rule on nuclear scales:
- Binds protons to neutrons inside nuclei
- Mediates radioactivity and nuclear reactions
Return to [
Unit 4 Index
|
Astronomy 161 Main Page
]
Updated: 2006 October 23
Copyright © Richard W. Pogge,
All Rights Reserved.

 [Click on image for full-size version (17Kb GIF)]
[Click on image for full-size version (17Kb GIF)]