

|
Astronomy 161:
An Introduction to Solar System Astronomy
Prof. Richard Pogge, MTWThF 9:30
|
Lecture 29:
The Earth's Atmosphere
Key Ideas:
Composition:
- Nitrogen, Oxygen, Argon & Water Vapor
- Lack of Hydrogen or Helium
Greenhouse Effect:
- Explains why the Earth is as warm as it is.
Structure of the Atmosphere
Origin of the Atmosphere
- Primordial atmosphere: mostly CO2
- Evolution of the atmosphere
The Earth's Atmosphere
Composition:
- 77% N2 (molecular nitrogen)
- 21% O2 (molecular oxygen)
- 1% H2O (Water Vapor)
- 0.93% Argon
- CO2 (0.035%)
- Traces of CH4 (methane), Inert Gases (Ne, He,
Kr, Xe)
- Particulates (silicate dust, sea salt, sulfates, etc.)
Why is there so little Hydrogen?
Hydrogen & Helium are the most abundant elements in the
Universe, yet they are very rare in the Earth's atmosphere.
Why?
- H & He are small and light, and so moves very fast at
a given atmospheric temperature.
- The mean speeds are greater than the escape
velocity from the Earth.
As a consequence, most of the H and He escaped from the atmosphere
a long time ago.
The Earth is too small to retain atmospheric H & He.
Why is the Earth so warm?
If there was no atmosphere, the Earth's temperature could
be calculated by balancing:
- The energy in sunlight absorbed by the Earth
- The energy radiated as infrared photons by the warm Earth.
Equilibrium Temperature should be T=260 K
- Water freezes at 273 K, so
- You would expect no liquid water
Why is this not the case? In other words, why is the Earth as
warm as it is?
Where does all the sunlight go?
The Earth's atmosphere strongly absorbs infrared and UV
radiation:
- Absorbs IR & UV photons direct from the Sun
- Absorbs IR photons radiated by the ground
- Clouds can reflect or scatter sunlight.
The ground absorbs sunlight
- Absorbs UV, visible, and IR photons.
- Some light is reflected (clouds, ground, snow etc.)
The "Energy Budget" for Sunlight:
- 45% is absorbed by the ground (heats the ground)
- 22% is absorbed in the atmosphere (heats the air)
- 26% is reflected back into space by clouds and lost
- 7% is reflected back into space by the ground, oceans, snow & ice.
Only 67% of sunlight actually heats the Earth.
Greenhouse Effect
The atmosphere is transparent to visible light, but mostly opaque to
infrared.
Infrared "opacity" comes from absorption bands of
H2O, CO2, CH4 and others molecules.
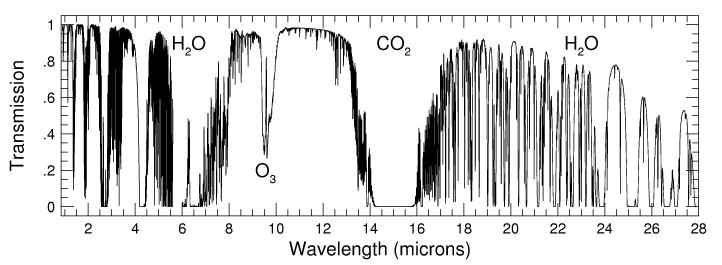
- Infrared Absorption of the Atmosphere from 1-28 microns (calculated)
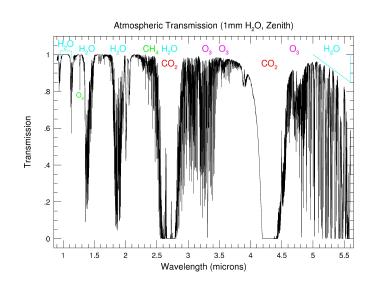
- Zoom into the near-Infrared (1-6micron) showing specific molecular bands
Visible Sunlight passes through the Earth's atmosphere to the
ground:
- Photons are absorbed by the ground, heating it up
- The warm ground radiates infrared photons (Wein's Law)
The atmosphere, however, is mostly opaque to infrared photons:
- Most of the infrared photons emitted by the warm ground
get absorbed by the atmosphere on their way out, heating the atmosphere.
The Greenhouse Effect is responsible for making the Earth
about 35K warmer than it would be if there it had no atmosphere.
The implications are very interesting: Without the Greenhouse Effect,
there would be no liquid water on the Earth, only ice. Since life as we
understand it requires liquid water, if there was no Greenhouse Effect,
the Earth would be inhospitable to life.
Note:
The term "greenhouse effect" is something of a misnomer.
Greenhouses (glass buildings mean to stay warm through winter for
growing plants) work primarily by inhibiting convection (boiling motions
of air warmed by solar-heating of the ground inside the greenhouse),
rather than by radiative trapping of sunlight behind glass. Radiative
trapping does contribute to how a garden greenhouse works, but
only to a fraction of the total heating experienced inside a greenhouse.
Atmospheric Pressure
Weight of air above makes the surface pressure higher.
The atmosphere's pressure falls as you climb in altitude:
- Pressure drops 50% for each 5.5 km of altitude
- At Sea Level: ~1 kg/cm2 (14 pounds/inch2)
For example, at the summit of Mt. Everest at 8850 meters, the air
pressure is about 1/3 what it is at sea level.
Structure of the Atmosphere
The Earth's atmosphere is divided vertically into several thermal layers,
from lowest to highest above the ground:
- Troposphere - weather layer
- Stratosphere - heated by UV absorption, primarily
in the ozone (O3) layer
- Mesosphere - cooler intermediate region
- Thermosphere - heated by UV & X-ray photons
The atmosphere does not so much stop as get thinner until it is
nearly indistinguishable from interplanetary space.
Origin of the Earth's Atmosphere
After losing most of its original H and He, the Primordial
Atmosphere of the Earth was built up by outgassing of the
crust by volcanos:
- Mostly H2O and CO2
- Small amounts of N2 and sulfates
- No oxygen (O2).
This is very different than our present atmosphere. How
did our atmosphere get the way it did?
Where did all the CO2 go?
The primordial atmosphere had ~1000 times more CO2 than it does now.
Where did it all go?
- H2O condensed to form the oceans.
- CO2 dissolved into the oceans and
precipitated out as carbonates (e.g., limestone).
Most of the present-day CO2 is locked up in crustal rocks
and dissolved in the oceans.
By contrast, N2 is chemically inactive
- It stayed a gas in the atmosphere and become its dominant constituent.
Where did the O2 come from?
The second major constituent of the present-day atmosphere is
Oxygen (O2), but it was absent in the Primordial
Atmosphere. Where did all the O2 come from?
- Molecular Oxygen (O2) comes primarily from
photosynthesis in plants and algae.
- The O2 content of the atmosphere has increased
from 1% to 21% during the past 600 Myr.
Ozone (O3):
- Forms in stratosphere from O2 interacting with solar UV photons.
- Blocks UV photons from reaching the ground.
This made land life possible as solar UV radiation is hazardous
to life.
The presence of O2 and O3 in our atmosphere is
a sign of life (photosynthesis).
Atmospheric Evolution
Atmospheres are complex, dynamic systems that evolve over
time.
Past Evolution:
- Condensation of H2O into the oceans.
- Locking up of CO2 into carbonaceous rocks
- Formation of O2 by photosynthesis in plants & algae
This evolution continues into the present day.
- CO2 content of the atmosphere is regulated by a complex
balance cycle.
- Increases in O2 and methane (CH4) from "biomass"
(plants and animals)
- Human activity (fuel burning & agriculture)
Human Impact on the Atmosphere
The impacts of human activity on the Earth's atmosphere include:
- Increasing amounts of atmospheric greenhouse gases, in
the amount of 20 billions tons/year, from industrial and
agricultural activity.
- Ozone layer destruction by industrial emission of
Chlorofluorocarbons (CFCs).
- Increasing particulates from a combination of industrial pollution,
cooking fires, and rainforest burning.
The impact of human activity (industrial or otherwise) upon the
Earth's atmosphere is real and it is measurable.
What is currently being debated, both scientifically and politically,
is the long-term implications of this impact, and what it may mean
for the future evolution of our atmosphere. This topic is formally beyond
the scope of this class, but of great general interest.
Return to [
Unit 5 Index
|
Astronomy 161 Main Page
]
Updated: 2006 October 28
Copyright © Richard W. Pogge,
All Rights Reserved.

