

|
Astronomy 161:
An Introduction to Solar System Astronomy
Prof. Richard Pogge, MTWThF 2:30
|
Lecture 24: Matter & Light
Key Ideas:
Temperature (Kelvin Scale)
- Measures internal energy content.
Kirchoff's Laws of Spectroscopy:
- A hot, dense object produces a continuous spectrum(blackbody spectrum).
- A hot, low-density gas produces an emission-line spectrum.
- A cool, dense gas produces an absorption-line spectrum.
The Interaction of Light & Matter
Light & Matter can interact in a number of different ways:
- Matter can transmit light (glass, water).
- Matter can reflect light.
- Matter can gain energy by absorbing light.
- Matter can lose energy by emitting light.
The last two (absorption and emission) bear on the internal
energy of the matter.
Temperature
Temperature is a measurement of the internal energy content of
an object.
- Solids:
- Higher temperature means higher average vibrational
energy per atom or molecule.
- Gases:
- Higher temperature means more average kinetic energy
(faster speeds) per atom or molecule.
Absolute Temperature
- At high temperatures:
- Atoms & molecules move very rapidly.
- At cooler temperatures:
- Atoms & molecules move more slowly.
If it gets cold enough, all motion will cease. How cold is
"cold enough"?
- Corresponds to a temperature of -273° Celsius (-459° F).
- Called "Absolute Zero".
Realistically, this represents an ultimate lower limit that is
physically unobtainable (you can get close, but you never get
exactly to it).
Kelvin Temperature Scale
An absolute temperature system in which the temperature
is directly proportional to the internal energy.
- Developed by British physicist William Thomson, First Lord Kelvin (19th century)
- Uses the Celsius degree, but a different zero temperature
Kelvin Absolute Temperature Scale (K):
- 0 K = Absolute Zero
- 273 K = pure water freezes (0° Celsius)
- 373 K = pure water boils (100° C)
The principal advantage of the Kelvin scale compared to the more
familiar Celsius and Fahrenheit scales is that temperature measured in
Kelvins is directly proportional to the amount of internal energy in an
object. If you double the internal energy, you double the temperature
in Kelvins. This is why the Kelvin scale is said to measure
absolute temperature. Both the Celsius and Fahrenheit systems
are difficult to use for relating the absolute energy content of objects
because they are tied arbitrarily to the freezing and boiling points of
water on the surface of the Earth.
We will primarily use the Kelvin scale in this course (and Astronomy
162), but will occasionally use Celsius (with Fahrenheit equivalents)
where we are talking about planetary temperatures.
What is a Spectrum?
A spectrum is the distribution of photon energies coming from a light
source:
- How many photons of each energy are emitted by the light source?
Spectra are observed by passing light through a spectrograph:
- Breaks the light into its component wavelengths and
spreads them apart (dispersion).
- Uses either prisms or diffraction gratings.
Kirchoff's Laws of Spectroscopy
- A hot solid or hot, dense gas produces a continuous spectrum.
- A hot, low-density gas produces an emission-line spectrum.
- A continuous spectrum source viewed through a cool, low-density gas produces
an absorption-line spectrum.
German physicist Gustav Kirchoff (1824-1887) formulated these laws
empirically during the mid-19th century. While they adequately describe
the different kinds of spectra that are observed, they do not explain
why these spectra appear in these circumstances. A physical explanation
had to wait until the 20th century for the development of quantum
mechanics and modern atomic theory.
Black Body Radiation
A Blackbody is an object that absorbs all light.
- Absorbs at all wavelengths.
As it absorbs light, it heats up.
- Characterized by its Temperature.
It is also the perfect radiator:
- Emits at all wavelengths (continuous spectrum)
- Energy emitted depends strongly on the Temperature.
- Peak wavelength also depends on Temperature.
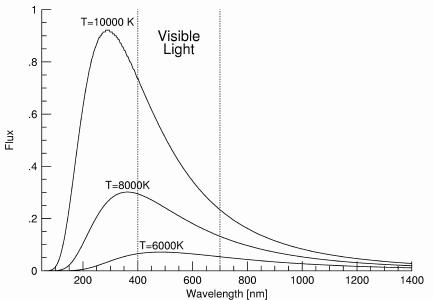
- Blackbody Spectra for three Temperatures: 10000K, 8000K, and 6000K.
Energy emitted per second per area by a blackbody with
Temperature (T):
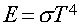
s is Boltzmann's constant (a
number).
In Words:
- "Hotter objects are Brighter at All Wavelengths"
Note the relative brightnesses at each wavelength for the three
different-temperature blackbodies above.
Wien's Law
Relates peak wavelength and Temperature:
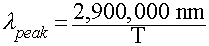
In Words:
- "Hotter objects are BLUER"
- "Cooler objects are REDDER"
Note the wavelength of peak brightness for the three
different-temperature blackbodies above.
Examples
Person: Body Temperature = 310 K
Sun: surface temperature = 5770 K
Emission-Line Spectrum
A hot, low-density gas, in which the atoms are relatively
isolated from each other, will emit an emission-line spectrum:

- Only emits light at particular wavelengths, giving the appearance of
bright, discrete emission lines.
- There is no light emitted between the emission lines.
19th chemists century noticed that each element, heated into
an incandescent gas in a flame, emitted unique emission lines.
- Mapped out the emission-line spectra of known atoms and
molecules.
- Used this as a tool to identify the composition of
unknown compounds.
- They did not, however, understand how it worked.
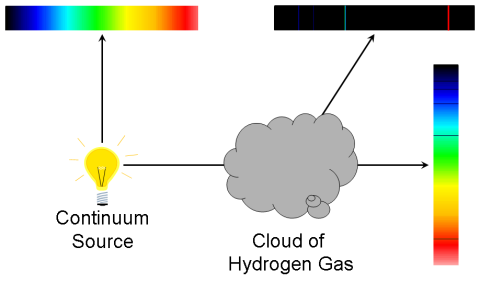
Absorption-Line Spectrum
Light from a continuous spectrum through a vessel containing a cooler
gas shows a continuous spectrum from the lamp crossed by of dark
absorption lines at particular wavelengths.:

- The wavelengths of the absorption lines correspond exactly
to the wavelengths of emission lines seen when the gas is hot!
- Light is being absorbed by the atoms in the gas.
Why does it work?
Why does each element have a characteristic line spectrum?
Answer:
- It is a reflection of the detailed structure of the atom.
- Depends on the number and arrangement of electrons in
orbit around the nucleus.
Discovering the reason unlocked the secret of the atom.
Return to [
Unit 4 Index
|
Astronomy 161 Main Page
]
Updated: 2007 October 19
Copyright © Richard W. Pogge,
All Rights Reserved.

