

|
Astronomy 161:
An Introduction to Solar System Astronomy
Prof. Richard Pogge, MTWThF 2:30
|
Lecture 25: Measuring Light:
Spectroscopy
Key Ideas:
Every atom, ion, and molecule has a unique spectral
signature.
- Reflection of their the underlying electron orbital
structure.
Absorption and Emission of Photons
- Excitation and De-Excitation
Ionization
- Remove one or more electrons, or add an extra electron.
Looking inside the Atom
Electrons cannot orbit just anywhere around a nucleus:
- Can only orbit in discrete orbitals.
- Each orbital corresponds to a particular energy of the
orbiting electron.
- If an electron does not have exactly the right energy, it
cannot be in that orbital (all or nothing).
The details are dictated by quantum mechanics.
Hydrogen: The Simplest Atom
An atom of Hydrogen (1H) consists of:
- A single proton in the nucleus.
- A single electron orbiting the nucleus.
The energy levels in the 1H atom look
schematically like this:
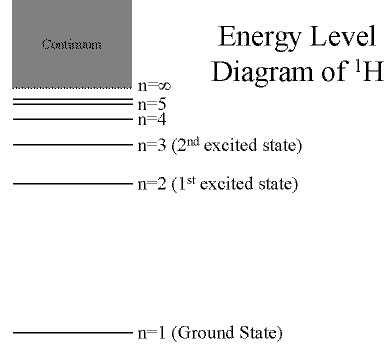
- (Click on the image to view at full scale [Size: 8Kb])
First orbital: Ground State (n=1)
- Lowest energy orbital the electron can reside in.
Higher orbitals: Excited States (n=2,3,...)
- Higher orbits around the nucleus.
- Come at specific, exact energies.
Emission & Absorption Lines
- Emission Lines:
- When an electron jumps from a higher to a lower energy
orbital, a single photon is emitted with exactly the
energy difference between orbitals. No more, no less.
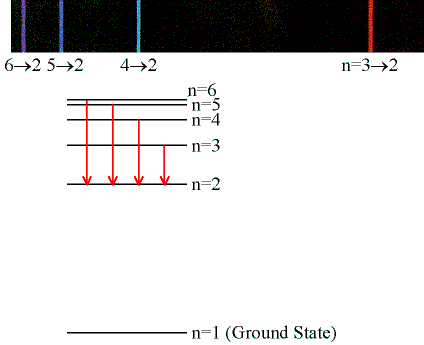
- (Click on the image to view at full scale [Size: 19Kb])
- Electrons can get into the excited states by either
- Colliding with other atoms or free electrons
- Absorbing photons of specific energies...
- Absorption Lines
- When an electron absorbs a photon with exactly the
energy needed to jump from a lower to a higher orbital.
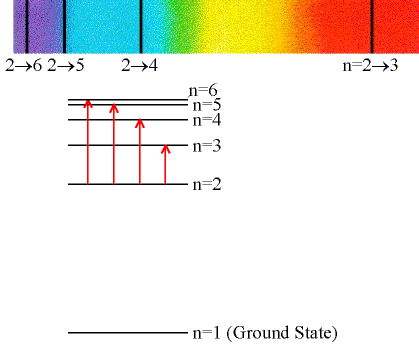
- (Click on the image to view at full scale [Size: 51Kb])
- Absorption is very specific:
- Only photons with the exact excitation energy are absorbed.
- All others pass through unabsorbed.
- The excited states decay by emitting photons in random directions.
Ionization
If an atom or molecule absorbs enough energy from a photon or
a collision, an electron can be ejected.
- Get a Positive Ion (atom or molecule with a
net positive charge).
Similarly, you can also add extra electrons:
- Get a Negative Ion (atom or molecule with a net negative charge).
Ions differ from their parent neutral atoms or molecules:
- Diferent spectral line signatures.
- Different chemical properties.
Fingerprinting Matter
Other atoms have more electrons, and hence more complex
electron orbital structures.
- Results in more complex line spectra.
- There is a unique spectrum for each element, reflecting
its unique electron orbital structure.
- Isotopes show the same lines, but slightly shifted in
wavelength.
Every element has its own, distinctive spectral signature.
Molecules
Molecules are more complex still:
- Compounds of two or more atoms, of the same or different elements.
- Share some electrons in common orbitals.
Results in very complex spectra:
- Broad "bands" consisting of many lines.
- Bands often span large wavelength regions.
Molecules mainly produce strong lines at infrared, microwave, and radio
wavelengths.
The Importance of Spectroscopy
From the emission or absorption lines in an object's spectrum,
we can learn:
- Which atoms and molecules are present, and in what proportions.
- Which atoms are ionized, and in what proportions.
- How excited (or not) the atoms are, tells us the objects state
(e.g., hot or cold).
These data give us a nearly complete picture of the physical
conditions in the object.
Spectroscopy is one of the most important tools of the astronomer.
Supplement
Want to explore spectroscopy? You can build a simple hand-held
spectrograph using common household items, plus a specially-purchased
(but inexpensive) diffraction grating:
- Build Yourself a Simple
Hand-Held Spectrograph
Return to [
Unit 4 Index
|
Astronomy 161 Main Page
]
Updated: 2007 October 19
Copyright © Richard W. Pogge,
All Rights Reserved.


