
|
Astronomy 161
Introduction to Solar System Astronomy
Prof. Paul Martini
|
Lecture 21: Matter
Key Ideas:
- Atoms are composed of
- a nucleus of protons and neutrons
- orbiting electrons
- Chemical Elements and Isotopes
- Radioactivity
- Four Fundamental Forces
- Gravitational and Electromagnetic
- Strong and Weak Nuclear Forces
Atoms
- Ordinary matter is found primarily in the form of atoms
- The word atom comes from the Greek word atomos, which means
indivisible
- Range of ordinary matter:
- Free subatomic particles (protons and electrons)
- Single atoms (hydrogen, helium, gold, etc.)
- Simple molecules (O2, H2O, etc.)
- Macromolecules (DNA, complex polymers, oil, etc.)
- Macroscopic objects (diamonds, trees, people, planets, etc.)
The Gold Foil Experiment
- Early models of the atom assumed atoms had uniform density
- In 1909 Hans Geiger and Ernest Marsden shot charged particles (called alpha particles) through a thin sheet of gold foil
- Surprisingly, about 98% went straight through, 2% were deflected, and 0.01% bounced straight back!
- The Atom was nearly empty!
Atomic Structure
- Nucleus of a heavy subatomic particles:
- proton: positively charged
- neutron: uncharged (neutral)
- Electrons orbiting the nucleus
- negatively charged particles
- 1/1836th the mass of a proton
- Atoms are mostly empty space
- Only 1 part in 1015 of space is occupied
- The rest of the volume is threaded by electromagnetic fields
Chemical Elements
- Atoms are separated into distinct Elements by the number of protons in
the nucleus
- Atomic Number:
- 1 proton: Hydrogen
- 2 proton: Helium
- 3 proton: Lithium
- Number of electrons = Number of protons
- All elements are Chemically Distinct
- An atom is the smallest division of a chemical element
Top Ten Most Abundant Elements
- 10) Sulfur
- 9) Magnesium
- 8) Iron
- 7) Silicon
- 6) Nitrogen
- 5) Neon
- 4) Carbon
- 3) Oxygen
- 2) Helium
- 1) Hydrogen
Variation in Abundance
- The average abundances of elements in the Universe need to be measured over a large Volume
- For example, the entire Solar System (at least)
- Local variations can be significant
- Oceans mostly Hydrogen and Oxygen (in water)
- Earth's crust mostly Oxygen, Silicon, Aluminum, and Iron
Known Elements
- 117 elements are currently known:
- 87 are metals
- 11 are gasses
- 2 occur as liquids (Bromine and Mercury)
- 26 are naturally radioactive
- 24 are made only in particle accelerators
- In addition, each element can have a number of different isotopes
Isotopes
- A given element can have many Isotopes
- Same number of protons
- Different number of neutrons
- Example:
- 12C has 6 protons and 6 neutrons
- 13C has 6 protons and 7 neutrons
- 14C has 6 protons and 8 neutrons
- These are chemically identical, but have different masses
Radioactivity
- Discovered by Antoine Becquerel in 1896, who observed that Uranium would fog photographic plates
- If a nucleus has too many or too few neutrons, it is unstable to radioactive decay
- Examples:
- 3H (1p + 2n) decays to 3He (2p + 1n) + 1 electron + 1 neutrino
- 14C (6p + 8n) decays to 14N (7p + 7n) + 1 electron + 1 neutrino
- This later reaction is the basis of radiocarbon dating
- Free neutrons are also unstable
- n decays to p + 1 electron + 1 neutrino
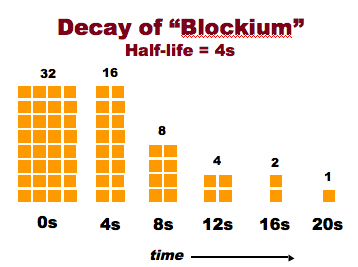
Radioactive Half-Life
- Radioactive decay is a random process
- The activity is measured by the Half-Life
- Time for half of the atoms to decay
- The more radioactive, the shorter the half-life
- Examples:
- 3H (1p + 2n) decays to 3He (2p + 1n) + 1 electron + 1 neutrino with a 12.26 year half-life
- 14C (6p + 8n) decays to 14N (7p + 7n) + 1 electron + 1 neutrino with a 5730 year half-life
- n decays to p + 1 electron + 1 neutrino with a 12 minute half-life
Uranium
- 238U has a half-life of 4.51 x 109 years. The vast majority of natural Uranium is in this isotope
- 235U has a half-life of 7 x 108 years. It is consequently very rare, but it produces lots of neutrons when it decays and can therefore sustain nuclear reactions (either for power or bombs)
Fundamental Forces of Nature
- All interactions in nature are governed by four "fundamental" forces
- Gravitational Force
- Electromagnetic Force
- Strong Nuclear Force
- Weak Nuclear Force
Gravitational Force
- Gravitation binds matter over long distances
- Long-range attractive force
- Weakest force of nature
- Obeys the Inverse Square Law of distance:
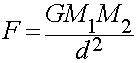
Electromagnetic Force
- Electromagnetic force acts between charged particles
- Like charges repel each other
- Opposite charges attract each other
- Long-range, inverse-square law force:
- Binds electrons and protons into atoms
- Binds atoms together into molecules
- Very strong:
- 1039 times stronger than Gravity
Strong and Weak Nuclear Forces
- Short-range forces (<10-15m) in atomic nuclei
- Strong force:
- Binds protons and neutrons into nuclei
- Strongest force of nature
- Weak force:
- Responsible for radioactivity (what turns a neutron into a proton, electron, and neutrino during neutron decay)
- Second strongest force
The Interplay of Forces
- Gravity rules on the largest scales:
- Binds massive objects together
- Controls orbital motions
- Electromagnetism rules on atomic scales
- Binds electrons to protons and atoms to atoms
- Mediates chemical reactions
- Strong and Weak Forces rule on nuclear scales
- Binds protons to neutrons inside nuclei
- Mediates radioactivity and nuclear reactions
See A Note about Graphics to learn
why some of the graphics shown in the lectures are not reproduced with
these notes.
[
Return to the Astronomy 161 Main Page
|
Unit 4 Page
]
Updated: 2010 January 30
Copyright © Paul Martini All Rights
Reserved.


