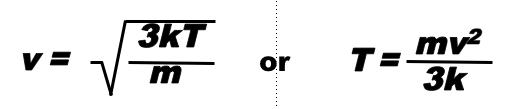
|
Astronomy 161
Introduction to Solar System Astronomy
Prof. Paul Martini
|
Lecture 27: The Earth's Atmosphere
Key Ideas:
- Physical processes
- Reflection, absorption, and energy transport
- Heat (energy) transport via Conduction, Convection, and Radiation
- Temperature, particle velocity, and mass
- Composition:
- Nitrogen, Oxygen, Argon, and Water Vapor
- Lack of Hydrogen and Helium
- Greenhouse Effect
- Structure of the Atmosphere
- Origin and Evolution of the Atmosphere
Reflection, Absorption, and Energy
- What is the impact of color on reflection and absorption?
- White surfaces are more reflective, black surfaces are more absorptive
- Reflection and absorption can depend on wavelength
- More energy absorption leads to more heating
Heat (Energy) Transport
Conduction: contact between solid materials
Convection: flows in liquids or gas
Radiation: from one surface to another (via photons)
For a pot on a stove, conduction heats the pot, convection heats the water, and radiation is felt from the sides
Temperature and Gas Velocity
The temperature of a gas particle is proportional to its mass times its velocity squared
E = 1/2 mv2 = 3/2 kT
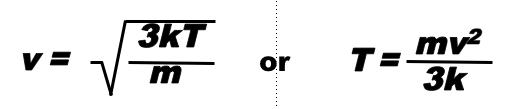 Lighter gas particles will move faster than heavier gas particles at the same temperature
For example, lighter elements like particles of Hydrogen gas will move faster than heavier particles like Oxygen molecules
Lighter gas particles will move faster than heavier gas particles at the same temperature
For example, lighter elements like particles of Hydrogen gas will move faster than heavier particles like Oxygen molecules
The Earth's Atmosphere
- Composition:
- 77% N2 (molecular nitrogen)
- 21% O2 (molecular oxygen)
- 1% H2O (water vapor)
- 0.93% Argon
- 0.035% CO2 (carbon dioxide)
- Traces of CH4, Inert gases (Ne, He, Kr, Xe)
- Particulates (silicate dust, sea salt, sulfates, etc.)
Why is there so little Hydrogen?
- Hydrogen and Helium are the most abundant elements in the Universe, yet they are very rare in the Earth's atmosphere. Why?
- H and He are small and light and so move very fast at a given atmospheric temperature
- Their mean atomic speeds are greater than the Earth's escape velocity (11.2 km/s)
- Most of the H and He escaped long ago
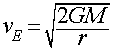
Earth is too low mass to retain atmospheric H and He
Why is the Earth so warm?
- If there was no atmosphere, the Earth's temperature could be found by balancing:
- The energy of sunlight absorbed by the Earth
- The energy radiated as infrared photons by the warm Earth
- Equilibrium Temperature: T = 260 K
- Water freezes at 273 K, so
- You would expect no liquid water!
Why is this not the case?
Where does all the sunlight go?
- 51% absorbed by the ground and oceans
- 19% absorbed by the atmosphere
- 30% reflected back into space
- The Earth's reflectivity or albedo is 30%
Greenhouse Effect
- The atmosphere is transparent to visible light but mostly opaque to infrared:
- Infrared opacity comes from absorption bands of H2O, CO2, CH4, and other molecules
- Sunlight heats the ground, warming it up:
- The warm ground radiates infrared photons
- These infrared photons are absorbed by the atmosphere, heating it
This makes the Earth ~35K warmer than it would be if there were no atmosphere
Atmospheric Pressure
- Atmospheric pressure drops with altitude:
- Sea Level: ~1 kg/cm2 (14 pounds/in2)
- Pressure drops 50% for every 5.5 km in altitude
- Mt. Everest
- Altitude: 8850m
- Pressure is 1/3 sea-level
Structure of the Atmosphere
- The Earth's atmosphere is divided vertically into several Thermal Layers
- Troposphere - lowest "weather" layer
- Stratosphere - heated by UV absorption in the ozone (O3) layer
- Mesosphere - cooler intermediate region
- Thermosphere - heated by UV and X-ray photons
- Above this the atmosphere merges smoothly into interplanetary space
The Origin of the Atmosphere
- After losing most of its H and He, the early atmosphere was built by outgassing from volcanoes:
- Mostly H2O and CO2
- Small amounts of N2 and sulfates
- No O2
- Very different from the present-day atmosphere
- How did it get the way it is now?
Where did all the CO2 go?
- The primordial atmosphere had ~1000 times more CO2 than it does now
- Where did it go?
- H2O rained out to form the oceans
- CO2 dissolved into ocean water and precipitated out as carbonates (e.g. limestone)
- Today most CO2 is locked up in crustal rocks and dissolved in the oceans
- N2 is chemically inactive
- Stays as the dominant constituent
Where did the O2 come from?
- Molecular Oxygen (O2) comes from life:
- Photosynthesis in plants and algae
- O2 content increased from 1% to 21% during the past 600 Myr
- Ozone (O3):
- Forms in the stratosphere when O2 interacts with solar UV photons
- Blocks UV from reaching the ground
- Made life on land possible
O2 and O3 are signs of life (photosynthesis)
Atmospheric Evolution
- Atmospheres are dynamic and evolving
- Past evolution
- Condensation of H2O into the oceans
- Locking up CO2 into carbonaceous rocks
- Formation of O2 by photosynthesis
- Continues into the present day:
- CO2 regulated by a complex cycle
- Increases in O2 and CH4 from "biomass"
- Human activity (fuel burning and agriculture)
Human Impact on the Atmosphere
- Primary Human Impacts:
- Emissions of greenhouse gases (20 billion tons per year) from industry and agriculture
- Ozone layer destruction by industrial CFCs
- Increase in atmospheric particulates (industrial pollution, cooking fires, and rainforest burning)
Human impact is real and measureable
Global Warming
- The Intergovernmental Panel on Climate Change
- Finds strong evidence for the increase in greenhouse gases and global warming
- Predicts the amount of future warming and other global climate change
- Bottom line: The Earth will warm by 0.2C per decade
This group shared the Nobel Peace Prize in 2007
Snows of Kilimanjaro
- Prof. Lonnie Thompson (OSU School of Earth Sciences) predicted ten
years ago that the snow will likely disappear within several decades
- Recent expeditions show this is happening, perhaps faster than expected
Implications
- The increase in greenhouse gases during the 20th century coincides with an 0.6 C rise in global mean temperature
- Is this caused by human activity?
- We are at least a major part of the cause
- Natural cycles are also in play
- Consequences of global warming are hard to predict accurately, but evoke concern because small changes could produce substantial negative consequences
See A Note about Graphics to learn
why some of the graphics shown in the lectures are not reproduced with
these notes.
[
Return to the Astronomy 161 Main Page
|
Unit 5 Page
]
Updated: 2010 February 14
Copyright © Paul Martini All Rights
Reserved.